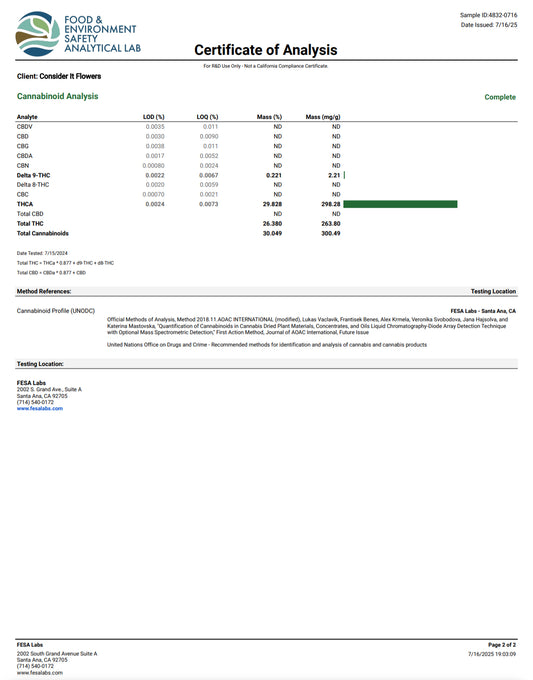
Featured products
Explore a wide selection of premium cannabis products designed to elevate your experience. From potent strains to carefully crafted edibles and top-quality extracts, discover the perfect option for your relaxation and enjoyment. Browse now for a diverse range of cannabis offerings that cater to different preferences and needs.
-
Molotov Cocktail (GH) - smalls 29.82%
Regular price From $20.00Regular priceUnit price / per$22.50Sale price From $20.00Sale -
Hash Burger (GH) 29.79%
Regular price From $20.00Regular priceUnit price / per$25.00Sale price From $20.00Sale -
Trop Cherry (GH) 27.76%
Regular price From $20.00Regular priceUnit price / per$25.00Sale price From $20.00Sale
Compliance Statement
Avondale Apothecary products are in compliance with federal law and there for legal according to the
following:
The 2018 Farm Bill & USDA Final Rule
In December of 2018, the 2018 Farm Bill was signed into law. It removed hemp, defined as cannabis (Cannabis Sativa L.) and derivatives of cannabis with extremely low concentrations of the psychoactive compound delta 9-tetrahydrocannabinol (THC)(no more than 0.3 percent THC
on a dry weight basis), from the definition of marijuana in the Controlled Substances Act (CSA).
The 2018 Farm Bill defines hemp as “any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers with no more than a .03% concentration of delta-9”.
Additionally, Avondale Apothecary complies with the USDA Final Rule , which indicates that a hemp crop is considered to be compliant if it is tested no more than thirty (30) days prior to harvest using a postdecarboxylation method and the results show that the delta-9 THC value does not exceed 0.3% on a dry weight basis:
“Postdecarboxylation. In the context of testing methodologies for THC concentration levels in hemp, means a value determined after the process of decarboxylation that determines the total potential delta-9 tetrahydrocannabinol content derived from the sum of the THC and THC-A content and reported on a dry weight basis. The postdecarboxylation value of THC can be calculated by using a chromatograph technique using heat, gas chromatography, through which THC-A is converted from its acid form to its neutral form, THC. Thus, this test calculates the
total potential THC in a given sample. The postdecarboxylation value of THC can also be
calculated by using a high-performance liquid chromatograph technique, which keeps the THC-A intact, and requires a conversion calculation of that THC-A to calculate total potential THC in
a given sample.”
The postdecarboxylation value for THC, as described above, is synonymous with the Total THC
in a hemp sample. For this reason, it is clear that a pre-harvested hemp crop is not compliant
unless its Total THC concentrations do not exceed 0.3% on a dry weight basis. [5]
Total THC Is Not Applicable to Harvested Hemp
The USDA Final Rule [6] only governs hemp production and not the regulation of hemp
distribution or hemp products which are governed by the Food and Drug Administration Farm Bill. Therefore, after post-harvest, the USDA has no further jurisdiction over the hemp crop leaving the Food and Drug Administration (FDA) as the only other agency with the authority to
regulate the hemp crop. The sole authority on post-harvested hemp is the Farm Bill and its definition specifically defines hemp in terms of its delta-9 THC concentration, not its Total THC content.
One of the key aspects that allows for a higher concentration of THC-A is that during the thirty (30) day harvest window in which a hemp crop must be tested for THC concentrations, the plant’s cannabinoid concentrations, including THC, continue to increase. [8] For this reason, pre-harvest hemp which has been tested and determined to be compliant may have total THC concentrations that exceed 0.3% when harvested. With that being said, the hemp crop will
remain compliance with FDA’s 2018 Farm Bill regulations as long as its delta-9 THC concentration does not surpass 0.3%
DEA Regulation of Hemp and Hemp Products
On August 21, 2020, the DEA published its Interim Final Rule (IFR) to further clarify that hemp
and hemp products are not controlled substances.
In order to meet the definition of “hemp”, and thus qualify for the exemption from Schedule I,
the derivative must not exceed the 0.3% delta-9 THC limit. The definition of “marihuana”
continues to state that “all parts of the plant Cannabis Sativa L.” and “every compound manufacture, salt, derivative, mixture, or preparation of such plant,” are Schedule I controlled substances unless they meet the definition of “hemp” by falling below the 0.3% delta-9 THC
limit on a dry weight basis.
This ruling confirms that products distributed by Avondale Apothecary are not controlled substances as long as their delta-9 THC concentrations do not exceed 0.3% on a dry weight basis.
Lawful Transportation of Hemp Products in Interstate Commerce
The 2018 Farm Bill legalized industrial hemp at the federal level and included a provision that
makes it illegal for states to prohibit the interstate transportation of hemp and hemp products as
follows:
No State or Indian Tribe shall prohibit the transportation or shipment of hemp or hemp products
produced in accordance with subtitle G or the Agricultural Marketing Act of 1946 (AMA (as
added by section 10113) through the State or the territory of the Indian Tribe, as applicable.
This ruling makes it clear that Avondale Apothecary is allowed to transport and ship hemp or hemp products as long as the products are produced in compliance with the 2018 Farm Bill by not exceeding a delta-9 THC concentration of 0.3% on a dry weight basis. Additionally, Avondale Apothecary products will not be shipped to states with regulations that have enacted a “Total THC” testing requirement imposing limitations on the requirements set out in the 2018 Farm Bill.
Avondale Apothecary Federal Product Compliance
Based on the 2018 Farm Bill, USDA Final Rule, and DEA regulations, Avondale Apothecary’s
products which contain delta-9 THC concentrations do not exceed 0.3% and are not controlle substances under Federal law. The above rules and regulations specifically define hemp in terms of its delta-9 THC concentrations, not its Total THC content meaning Avondale Apothecary products are in full compliance for retail sales and distribution.